A System Reaches Chemical Equilibrium When
A solution at equilibrium is. Reaching Equilibrium and the Equilibrium Position.

8 2 Chemical Equilibrium Chemistry Libretexts
Suppose you start out in a rigid container.

. When a chemical system is at equilibrium ____________________. The equilibrium between reagents and the products is achieved. Key Concepts and Summary.
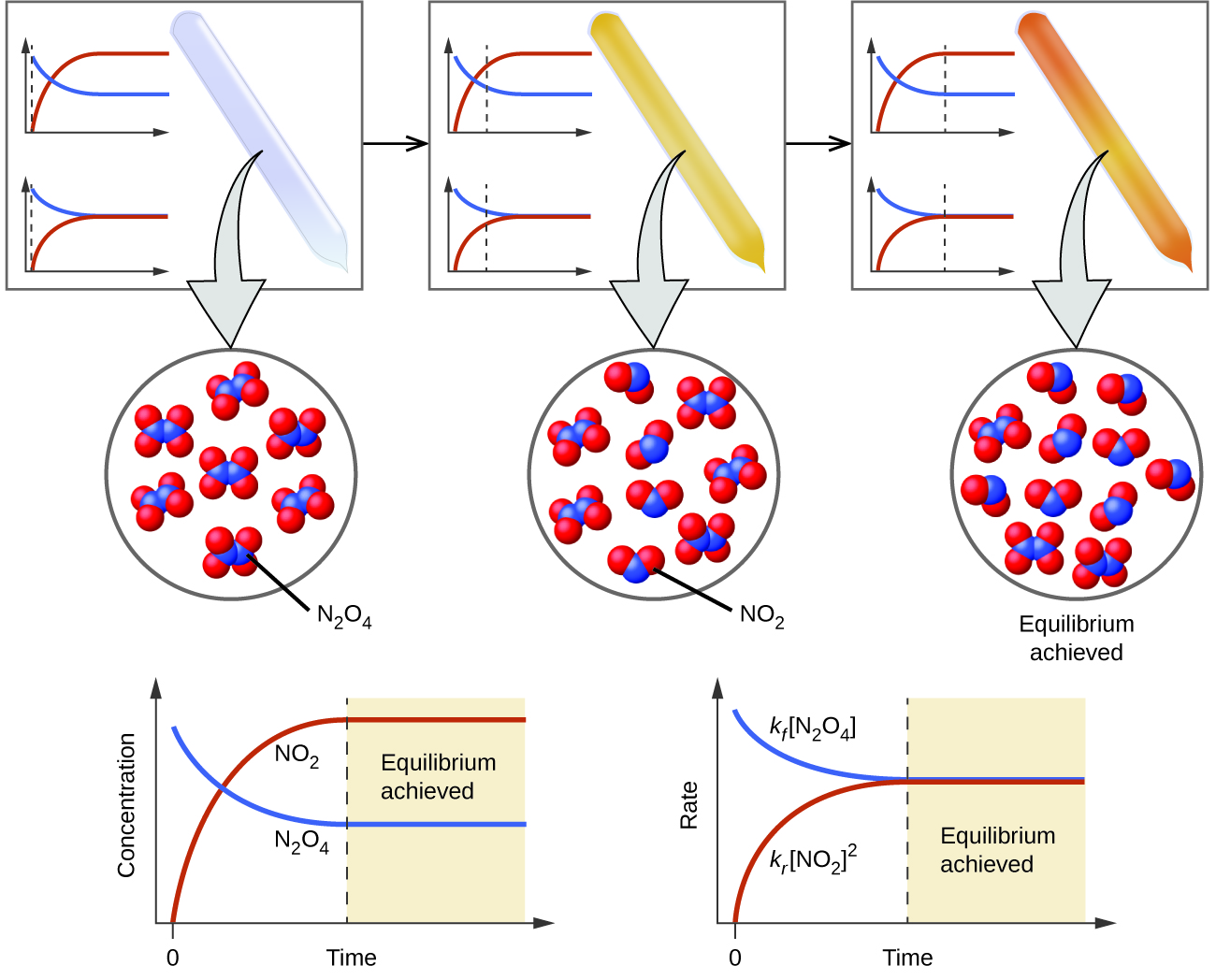
For homogeneous systems the equilibrium constant expression contains a term for. When the rate of the forward reaction equals the rate of the reverse reaction the system reaches chemical equilibrium. Q C c D d A a B b for the general balanced chemical equation a A b B c C d D.
A system reaches chemical equilibrium when a. 1 Which phrase defines chemical equilibrium. The reverse reaction no longer occurs in the system.
The ratio of product concentration to reactant concentration is 11 The reaction quotient is 1 The equilibrium constant is 1 The rate constant of the forward reaction equals the rate constant of the reverse reaction The reaction quotient is. When there is no further change in the concentrations of the reactants and the products due to the equal rates of the forward and reverse reactions at the time point of time the system is said to be in a dynamic state of equilibrium. Use the dissociation reaction to answer the question.
Chemistry questions and answers. At a given temperature the equilibrium composition is related to the. Equilibrium Constant When forward and reverse reactions occur at the same rate the system reaches dynamic equilibriumChemical equilibrium occurs when dynamic equilibrium realizes for ALL steps of the reaction ie.
2Use the table of data for a decomposition reaction to answer the question. No new product is formed by the forward reaction. Reversible reaction - a chemical reaction that proceeds.
Chemical equilibrium is the state of a system in which the concentration of the reactant and the concentration of the products do not change over time and the systems properties do not change. Mar 13 2022. A B A B.
When the rate of the forward reaction is equal to the rate of the reverse reaction the state of chemical equilibrium is achieved by the system. Chemical equilibrium is said to be achieved by the system when the rate of the forward reaction is equal to the rate of the reverse reaction. A reaction is at equilibrium when the amounts of reactants or products no longer change.
Concentration C at T1 gL C at T2 gL C at T3 gL C at T4 gL Reactant 300 100 50 50. FeSCN2 aq Fe3 aq SCN- aq In the reaction FeSCN2 ions are red Fe3 ions are pale yellow and SCN- ions are colorless. In a chemical reaction chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time so that there is no observable change in the properties of the system.
The equilibrium constant Kp is 48 x 10-31 at 25C for N2 g O2 g 2NO g. What is the equilibrium constant for this reaction at this temperature. The rate at which the forward reaction occurs equals the rate of the reverse reaction.
We say that the iodine is distributed between the two phases. The concentration of reactants in the system is equal to the concentration of products. Even though chemical reactions that reach equilibrium occur in both directions the reagents on the right side of the equation are assumed to be the products of the reaction and the reagents on the left side of the equation are assumed to be the reactants.
This state results when the forward reaction proceeds at the same rate as the reverse reaction. All chemical equilibria are dynamic equilibria. What is true about a system in chemical equilibrium.
Chemical equilibrium is the condition which occurs when the concentration of reactants and products participating in a chemical reaction exhibit no net change over time. What are the partial pressures of each gas when the system reaches equilibrium. A chemical system reaches equilibrium at the instant when the rate of the formation of products becomes zero.
The initial partial pressure of N2 675 and that of O2 472. Chemical equilibrium - the state of a reaction in which all reactants and products have reached constant concentrations in a closed system. When there is no further change in the concentrations of the reactants and the products due to the equal rates of the forward and reverse reactions the system is said to be in a state of dynamic equilibrium.
When a system consists of competing forward and reverse reaction rates the reaction will proceed until chemical equilibrium is reached. Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re-form reactants by the reverse reaction. Mn20 CO320 Mn2eq CO32eqM M M M d.
A dynamic equilibrium is an equilibrium in which the rates of forward and reverse processes are equal. The reaction N2 3H2 2NH3 reaches equilibrium with concentrations of N2 004 H2 007 and NH3 002. We are accustomed to representing.
The amount of reactant changes but over time remains about the same. Consider the following general reaction between A and B. Group of answer choices.
When the iodine concentrations become constant we say that the system has reached distribution equilibrium. At which time did the system first reach chemical equilibrium. In a chemical reaction one or more chemical substances reactants undergo a change to produce one or more new chemical substances products.
A state in which the forward and reverse reactions are proceeding at equal rates. The reaction rates of the forward. Chemical equilibrium may also be called a steady state reaction.
To determine whether a system has reached equilibrium chemists use a quantity called the reaction quotient A quantity derived from a set of values measured at any time during the reaction of any mixture of reactants and products regardless of whether the system is at equilibrium.

Physical Chemistry Which Graph Shows Chemical Equilibrium Chemistry Stack Exchange

No comments for "A System Reaches Chemical Equilibrium When"
Post a Comment